|
Impact of Detergents on Membrane Protein Complex Isolation
Yu-Chen Lee, Jenny Arnling Baath, Ryan M. Bastle, Sonali Bhattacharjee, Mary Jo Cantoria, Mark Dornan, Enrique Gamero-Estevez, Lenzie Ford, Lenka Halova, Jennifer Kernan, Charlotte Kurten, Siran Li, Jerahme Martinez, Nalani Sachan, Medoune Sarr, Xiwei Shan, Nandhitha Subramanian, Keith Rivera, Darryl Pappin, and Sue-Hwa Lin
Journal of Proteome Research, published online November 7, 2017
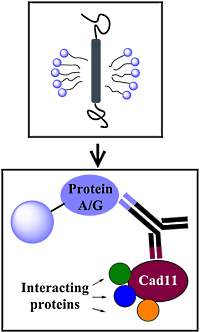
Detergents play an important role in the isolation of membrane protein complexes, affecting the native folding of the proteins, their binding to antibodies, and their interaction with partner proteins. In this paper, the authors investigated the effect of several commonly used detergents on Cad11 protein complex solubilization and coimmunoprecipitation.
Antibody pull-downs of the complex, coupled with iTRAQ quantitation, were used to determine the associating proteins. Seven detergents were examined and the authors found variations in the association of certain proteins with Cad11 depending on the detergents used.
DDM and Triton X-100 were found to be more efficient in solubilization and immunoprecipitation of Cad11 and provided 40 new candidate Cad11-interacting proteins. The authors suggest that using a panel of detergents may lead to a more comprehensive view of the composition of complexes.
|
 |