|
Mining the human tissue proteome for protein citrullination
Chien-Yun Lee, Dongxue Wang, Mathias Wilhelm, Daniel Paul Zolg, Tobias Schmidt, Karsten Schnatbaum, Ulf Reimer, Fredrik Ponten, Mathias Uhlen, Hannes Hahne and Bernhard Kuster
Molecular & Cellular Proteomics, Published online April 2, 2018
Citrullination is the conversion of arginine to citrulline by peptidylarginine deiminases leading to the mass increment of 0.9840 Da. This mass is identical to the common deamidation of Asn/Gln residues, leading to ambiguity in database searching of data from complex digests.
Citrullination has been implicated in many cellular processes such as terminal epidermal differentiation, apoptosis, central nervous system (CNS) stability, immune response, gene regulation, and embryonic development.
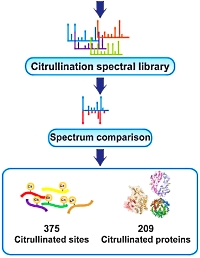
To address the sparsity of information on specific citrullination sites, the authors mined deep proteomes of 30 human tissues for endogenous protein citrullination. To analyze the ~70 million MS/MS spectra, generated by 1,116 LC-MS/MS runs, they used the neutral loss of isocyanic acid, the immonium ion of citrulline, manual spectrum interpretation, as well as reference spectra of synthetic peptides. This resulted in the identification of 375 citrullinated sites on 209 human proteins across 26 human tissues.
|
 |